The increasing complexity of modern trials demands more intensive data capture. Innovative study designs seek to minimize patient exposure to potential risks, improve convenience and shorten therapy time-to-market —requiring collecting and assimilating more data points than ever before. Data now comes in multiple structures from disparate sources increasingly needed in real-time.
Many sponsors and clinical organizations struggle with managing the many vendors that provide these critical data flows, limiting effective oversight since reporting is often lagging behind the pace of clinical conduct.
The Impact
Delayed data in clinical trials has an impact throughout the drug R&D lifecycle.
Clinical Operations
Instead of focusing efforts on operational management or data review, clinical teams are compelled to spend precious time seeking out, cleaning, and reconciling data. This drain on key study team members hinders their ability to see the big picture and results in critical decision-making delays. Many clinical researchers intuitively know that their clinical data issues result in trial delays but have little ability to make meaningful corrections since delayed data also limits timely issue resolution and course correction
Time is money. To grasp the reality of time spent on data delivery, encapsia surveyed biopharma and life sciences sponsors, contract research organizations (CROs), clinical technology providers, and patient recruitment/site management organizations. The results revealed that clinical operations personnel individually spend, on average, about 8 hours a week searching for, collating, and combining datasets. The raw math on costs per FTE alone should be the catalyst to substantively address your clinical data strategy from protocol to submission.
Business Operations
Senior management needs access to real-time data analysis and intelligence to be able to make key strategic decisions. When access to this data is delayed, the ability to make high level-decisions on funding rounds, study commencements, study expansions, and other operational activities is also delayed. This can lead to costly setbacks and significantly reduced decision effectiveness.
Site Operations
Many sponsors pay sites based on completed data, yet typically have extended delays in consolidating and assessing data and making protocol amendment requests. Consequently, some investigator sites are running trials at a deficit time while they wait for the sponsors to fully assess the data and make payments. This puts an extra burden on the sites that may affect study quality if they need to hire additional staff, equipment, patient reimbursement, or other budget items covered in their sponsor trial agreement. Some therapeutic areas also have continuous enrolment which may slow site data capture and query responsiveness which then delays SDV and site payment. Sites then may have to allocate their focus and resources on trials that are more favorably designed or office tools to enable faster payments. Finally and most importantly, patients are negatively affected, such as when delays make patient replacement necessary— rendering initial patient exposure and contribution in vain.
Why Exactly is Data Delayed?
A myriad of reasons is behind delayed data in the clinical trials space. In the encapsia survey, respondents shared why they can’t see data at the point it’s needed.
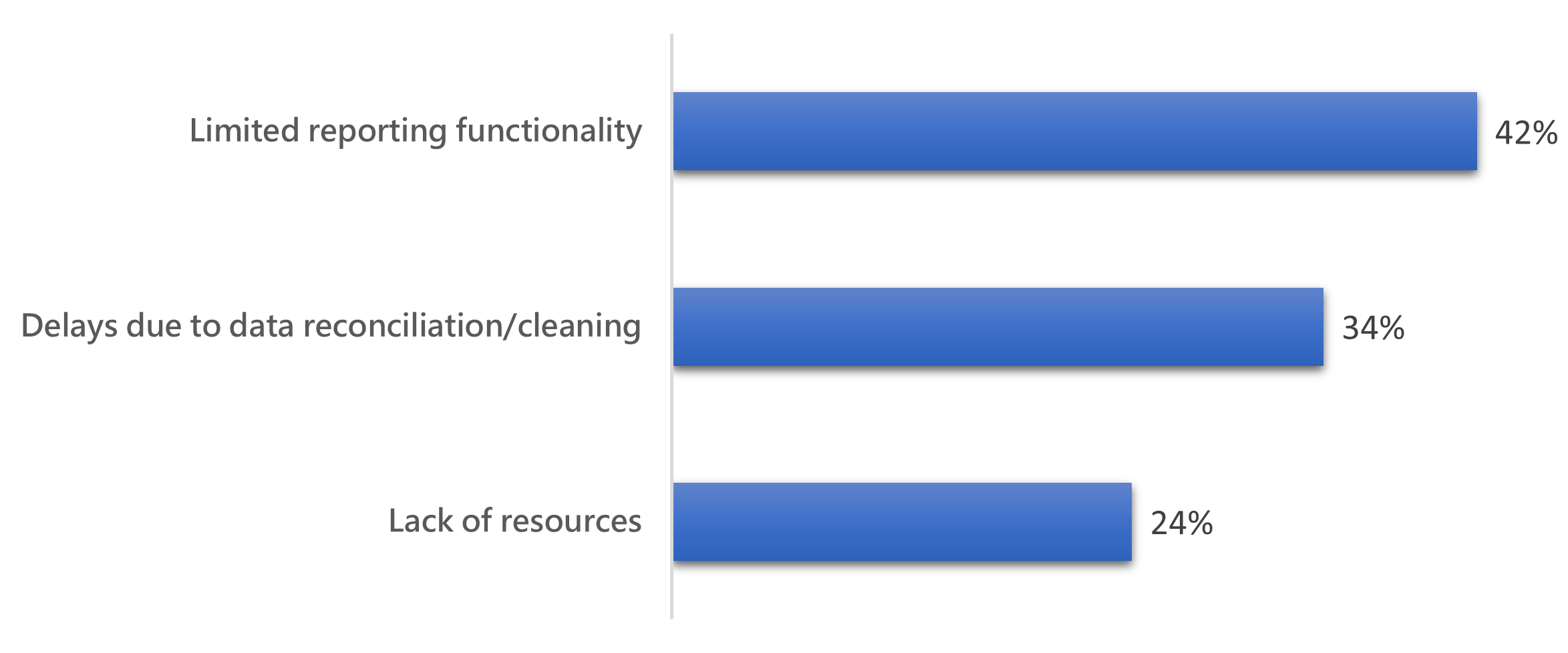
- 42% cited limited reporting functionality
- 24% cited a lack of resources
- 34% had delays due to data reconciliation/cleaning
Secondarily, the clinical industry is protocol and process-driven and tends to be reticent to embrace new technology. Despite advancements in clinical trial technology, many organizations are still heavily reliant on manual, error-prone data processing such as using Excel spreadsheets.
The Solution
Clinical researchers shouldn’t have to spend time and resources manually fixing data issues. Sponsors should be free of the burden of delayed data collation and assessment. Executives should have real-time views of data so they can make critical business decisions. Their primary mission is too important to be subject to arcane software and email threads.
Technology can and should be used to remove unnecessary manual processes, reduce risk, and eliminate delays so patients can get much-needed therapies on time.
Enter encapsia.
encapsia is a powerful and holistic clinical trial platform. It allows you to manage, explore, visualize, and analyze your clinical data more effectively, so you can make decisions that move your trials forward while saving resources and improving site satisfaction.
Learn how encapsia can help you!
To book a no-obligation meeting or demonstration please get in touch by using the contact form below:
Contact us

